Which of the Following Choices Is a Single Replacement Reaction
H2O CO2 H2CO3. In the equation 2Al s 3Fe NO 3 2 aq 3Fe s 2Al NO 3 3 aq iron has been replaced by.
Single Replacement Reactions Article Khan Academy
4 points Group of answer choices It is a single replacement reaction and all four compounds are different.

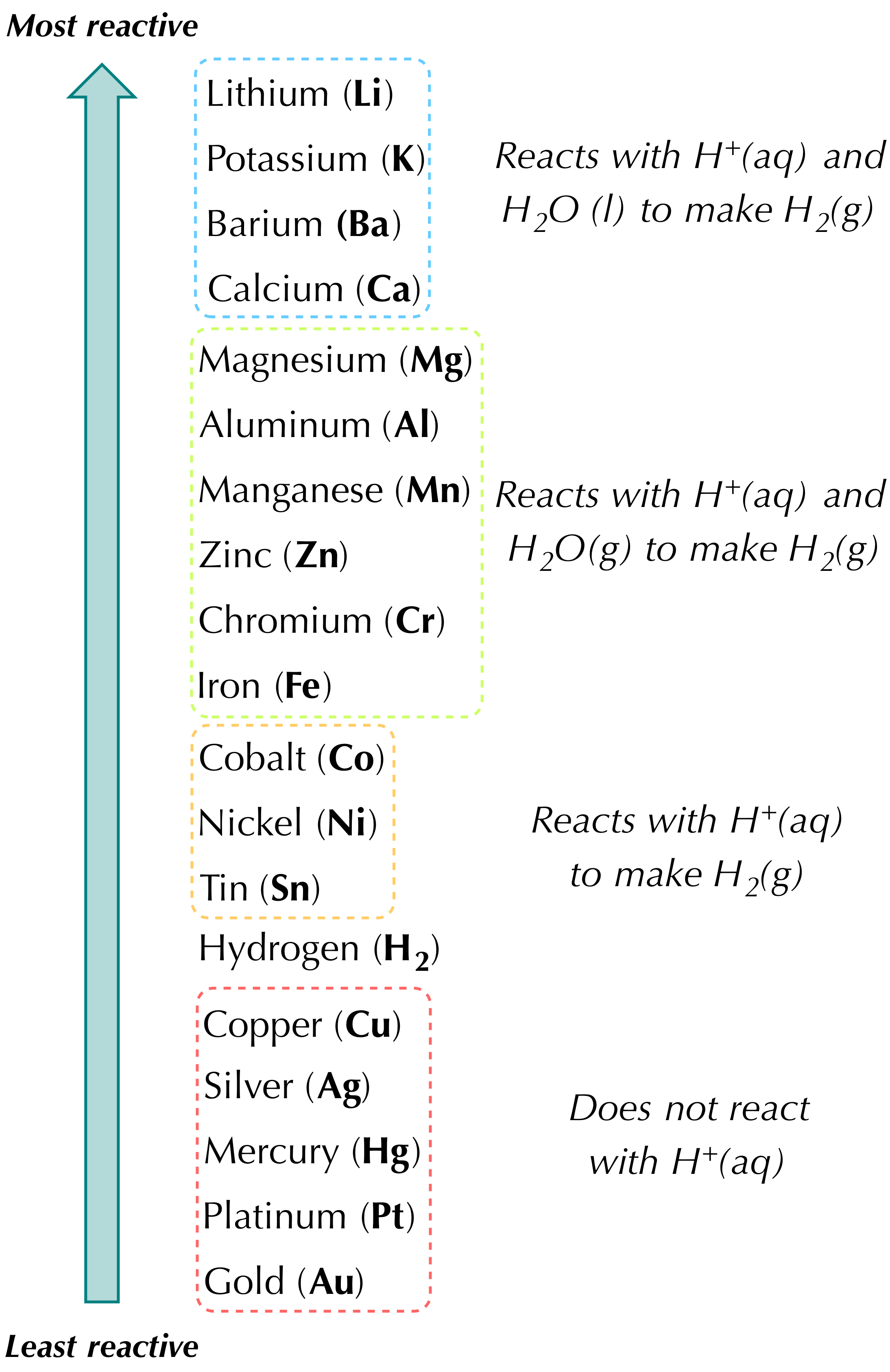
. A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. The more reactive element will substitute the less reactive element. A metal replaces another metal that is in solution.
C 5 H 10 O 4 O 2 -- CO 2 H 2 O. Fe 3NaBr FeBr3 3Na. -Cl2 g NaF aq ---Ks H2O l ---Al s HBr aq --.
What happen in a single. Ba s H2O no reaction Bao and H2 Ba OH2 and H20 Bao and H20 Ba OH2 and H2. Write a balanced equation for the reaction of aqueous solutions of potassium sulfide and hydrochloric acid.
CH4 2O2 CO2 2H2O. Which of the following is a single replacement reaction. There are two types of single replacement reactions.
Hydrogen usually forms the cation in a single replacement reaction. View the full answer. D Option D is the reaction colorredFe colorblueCuSO_4 rarr colorblueCu colorredFeSO_4 We can see from the color code that this is a single replacement reaction conforming to the first image above so option D is.
2 question The reaction 2Mgs O2g -- 2MgOs is a Group of answer choices double-replacement reaction. The substances listed on the left side of a chemical reactions are the. The correct answer is option d that is Zn H₂SO₄ ZnSO₄ H₂.
Single replacement reaction also known as single displacement reaction for example. Dear student A single replacement reaction is a chemical reaction in which a more reactive element replaces a less reactive eleme. Which of the following is true about this type of chemical reaction.
A single displacement reaction refers to a kind of chemical reaction in which one element substitutes another element in a chemical reaction. 20 Questions Show answers. This includes groups 1 and 2 some of group 13 and 14 elements and the transition metals.
The common non-metals in single replacement reactions are the group 17 elements which generally form anions with a 1- charge. Zn CuCl_2 Cu ZnCl_2 A halogen replaces another halogen that is in. When balancing equations a ____ can be placed to the left of a formula of a substance to make the equations balanced.
Identify the two single-replacement reactions that will occur. Which of the following is the general formula for a decomposition reaction. 2NaClO3 s 2NaCl s 3O2 g KCl aq AgNO3 aq KNO3 aq AgCl s Fe s CuSO4 aq FeSO4 aq Cu s 2NaHCO3 s Na2CO3 s CO2 g H2O g 2Fe s 3Cl2 g 2 FeCl3 s.
Ca OH2 H2SO4 CaSO4 2H2O. Chemistry QA Library Which of the following chemical reactions is classified as a single replacement reaction. Metals will usually form cations.
Which reaction type is the following. Abcacb so basically it means when an element replaces an element of a compound. There are two types of single replacement reactions.
A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. What are the products from the following single-replacement reaction. A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound.
A BC B AC Example. This leaves us with one final option. It is a double replacement reaction and all four compounds are different.
A metal replaces another metal that is in solution. It is a single replacement reaction and each compound has the same set of ions.

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube
No comments for "Which of the Following Choices Is a Single Replacement Reaction"
Post a Comment